About Tracking Technology


Online Tracking Assessment
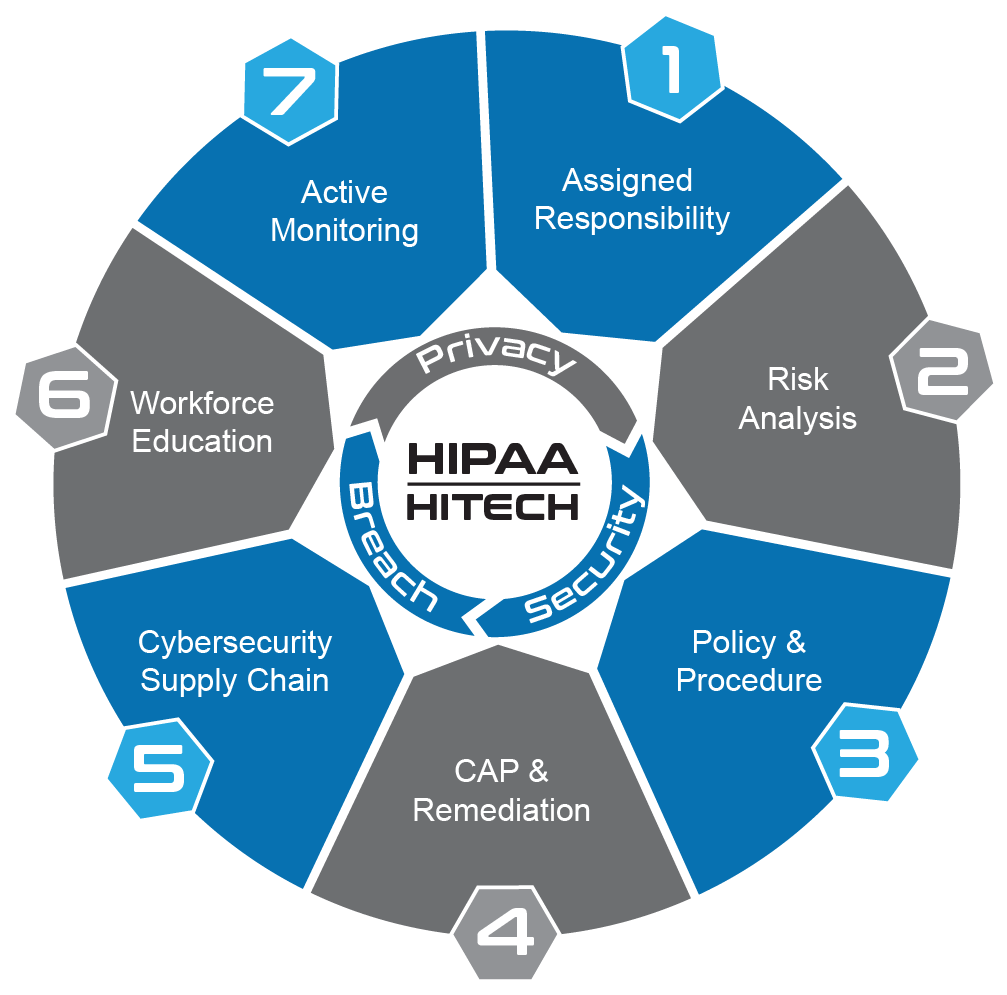
OCR Mandate for HIPAA Compliance
- Identify 3rd party resources across websites
- Evaluate 3rd party resources using fingerprinting or tracking technology
- Establish actionable recommendations
- Ensure HIPAA compliance with OCR guidance for online tracking
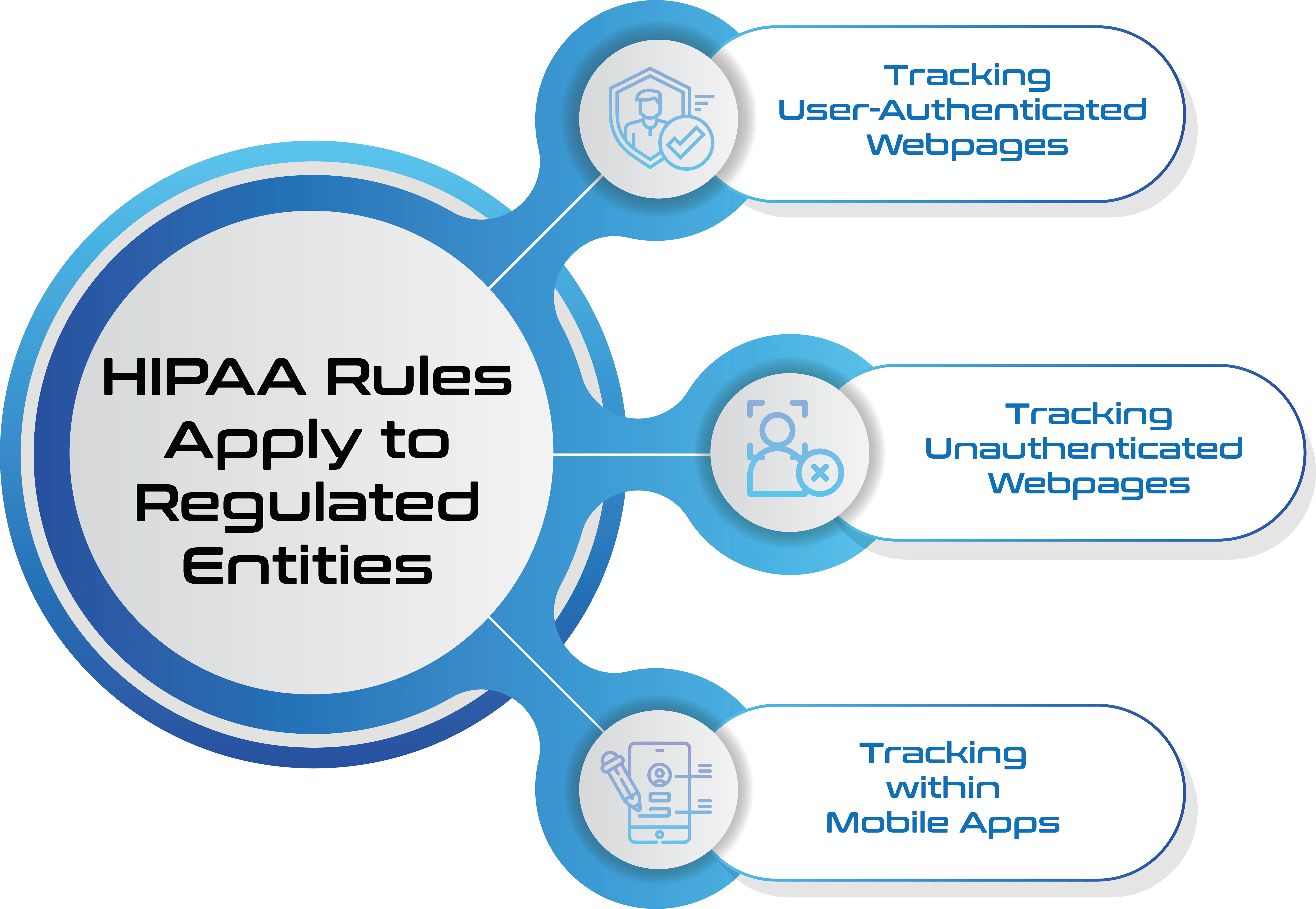
HIPAA Scope